Ammonia in an Aquarium: Is Your Tank Turning Toxic? Spot the Signs of Ammonia Poisoning in Fish!

Ammonia in an aquarium: Part 1
These are the 3 best ways to get rid of ammonia from your fish tank:
1. Add at least one more filtration system to your aquarium. This will at least double the amount of ammonia reducing bacteria in your aquarium.
2. Use massive water changes to remove the ammonia ladened water and replace it with fresh, clean water.
3. Wait. Move your fish to a second aquarium and then wait for the nitrifying bacteria in the first aquarium to grow abundant enough to lower the ammonia level to zero.
Ammonia is highly toxic to fish. The presence of ammonia can kill fish fairly quickly. I’ve seen this happen in as little as 24 hours. For example, about a year ago, a large snail I received died, and I didn’t see what had happened until one of my fish died out of the blue.
Yep, I have had the same frustrating aquarium keeping problems as you might have had.
One way to look at ammonia in an aquarium is to imagine that you are closed in a room filled with a strong concentration of ammonia in the air. Not a pleasant thought, is it? The gas would severely injure you if given enough time. Injuries could include blindness, skin burns, lung damage, and more. (https://www.mountsinai.org/health-library/poison/ammonia-poisoning )
So getting rid of ammonia naturally in an aquarium usually means increasing the nitrifying bacteria or changing out a large volume of water.
This might be a good luck / bad luck article. I suspect that if you’re reading this; you have an ammonia problem in your aquarium already (bad luck). That said, I’ll help you as much as I can for your sake and the sake of your fish (good luck).
So, you find ammonia in your aquarium. Why does this happen? It is always an inadequate amount of nitrifying bacteria in relation to the aquarium waste load. Waste load can be fish poop, fish pee (yes, they pee), uneaten food, rotting plant material and, occasionally, a fish that has died and that you haven’t noticed or found yet.
Discussing nitrogen cycling in a fish aquarium isn’t the reason I’m writing this article. There are plenty of other videos and articles that give excellent information about this. I will discuss ammonia regarding the nitrifying cycle of aquarium waste.
Ammonia is a naturally occurring chemical in an aquarium. It is a product of the decomposition of uneaten food, plants, and fish waste.
Nitrifying bacteria process ammonia into less harmful chemicals. Hence, our primary goal is to grow the amount of beneficial bacteria as quickly as possible.
Picture Above – My planted freshwater 2.9 gallon nano aquarium
Keeping fish can be costly, but there's a great way to offset those expenses.
Consider creating your own website, just like I did with this one!
I've used Divi to build this site, and it's incredibly user-friendly. With its drag-and-drop interface, creating your own website becomes a breeze. Simply tap this box to get started and see how easy it is with Divi.

For over four years, Divi has been my go-to website builder, and I can’t recommend it enough.
![]()
An 8 point guide to taking beautiful aquarium pictures

Solution #1 to get rid of ammonia in your aquarium naturally.
Add an extra filter system
Add at least one more filtration system to your aquarium. This will at least double the number of bacteria in your aquarium that gets rid of ammonia.
Adding an additional filter system is the best way to increase the good bacteria. The bacteria need a place to grow, and filtration systems are the perfect environment. I strongly recommend using two filter systems for each aquarium you are running. The reason for this is that if you damage the bacteria while cleaning one filter, the other filter can take up the slack for a while.
Article: How to care for your new fish bowl
“If you look closely, in the middle of the picture of my 2 gallon, seen through the water, is a black wire coming up from the heater. This heater lays flat underneath (not in) the aquarium bowl…”
Here are the filtration systems I use or have used:
1. A hang on or canister filter with hard and soft filter media. Soft media is usually polyester filter fiber (used to stuff pillows) in some form. Hard media comes in various shapes including balls, beads, and rings. Hard media can be made of plastic or ceramic. I use ceramic media formed as small, fat, one inch rings.
Be careful when cleaning your filter to protect the filter media and therefore protect the beneficial bacteria.The filter fiber will need to be replaced occasionally so having the hard media acts as an additional backup.
As an FYI, I never, ever clean my hard media. It doesn’t need it and the bacteria on it are too valuable to risk them going down the drain.
2. I’ve had outstanding success using sponge filters run by powerheads. The sponges are fantastic places for the bacteria to grow. Sponge filters can support so much growth they actually become clogged with good bacteria (and debris).
Make sure to be careful when cleaning the sponge filter so you don’t kill the bacteria. As with other filters, have a second filter running so that if you harm the bacteria when cleaning one sponge, the other can take up the ammonia load for a while.
3. Undergravel filters are amazing at growing beneficial bacteria. They work by pulling water down through the gravel, then up into the aquarium again. The gravel supports a huge load of nitrifying bacteria. For beginning aquarists, this would be a good and maybe best choice. I haven’t used an undergravel filter in years, though. They interfere with the growth of my aquarium plants.
Solution #2 to get rid of ammonia in your aquarium naturally.
Use massive water changes:
To save your fish, a 90 or 95% water change would not be too much.
Change a large percentage of the water in the tank daily. I would change out at least 90% of the water each time until your nitrifying bacteria catches up and can eliminate the ammonia naturally. Treat the water that goes back into the tank with a chlorine & chloramine remover made for aquarium. Your water company adds chlorine and chloramine to your tap water to kill bacteria. You can see why this would be a problem.
Discus
Discus Care

Solution #3 to get rid of ammonia in your aquarium naturally.
Remove the fish from your aquarium while you wait for the ammonia level to drop to zero:
Move the fish from the first tank into a container that is heated and oxygenated. Call this container your hospital tank. The fish can heal there while you fix the ammonia problem in your aquarium.
* Check the ammonia level in the new container daily. You don’t want the same problem in your hospital tank as you have in your first tank.
* Try hard to under-feed the hospitalized fish to keep the ammonia level down while they are in the new container. Personally, I would wait at least five or six days, if needed, before feeding the fish. At least until they are back in the old aquarium.
* Do water changes in the hospital container if or as needed.
In your old tank, you are now waiting for the numbers of nitrifying bacteria to become large enough to fix the ammonia problem. As mentioned earlier in this article, make sure you have at least two filter systems going in that tank.
* Check the old aquarium’s ammonia levels daily.
* When the ammonia reaches zero, put 10% of your fish back into your old aquarium.
* Check the ammonia in the aquarium the next day. If it is not zero, wait a day and check again. When the ammonia is zero, add a few more fish.
* Repeat the process until all of your fish are back in the aquarium.
* Keep two filtration systems going at all times in the aquarium for natural protection against ammonia in the future.
An aquarium filter that works and keeps on working. Period.
If you need to get an additional filtration system I have used the Fluval 207 canister filter on my 30 gallon tank for the last 18 months. It’s a champ. It just keeps going, doing what I bought it for.
Other buyers have given it 4.7 stars out of 5.
Here is a picture of mine:

* If you own a 10 to 30 gallon aquarium, click here.
* If you own a 30 to 45 gallon aquarium click here.
* If you own a 45 to 70 gallon aquarium click here.
Whether you are setting up a new aquarium or have one up and running already, always have at least two filter systems running. “An ounce of prevention is worth a pound of cure” couldn’t be more true than when discussing natural removal of ammonia in fish aquariums.
Now go make your fish happy and healthy, by getting rid of ammonia in your aquarium naturally.

My 30 gallon – male and female zebra danios
Signs of ammonia in your aquarium fish tank
“This might be a good luck / bad luck article. I suspect that if you’re reading this; you have an ammonia problem in your aquarium already (bad luck). That said, I’ll help you as much as I can for your sake and the sake of your fish (good luck).”
Other searches people that keep aquariums have done to find out about getting rid of ammonia:
How to avoid ammonia in the aquarium? Answer:
-
- Stop overfeeding your fish. Less uneaten food and less fish poop means less ammonia.
- Under stock your aquarium. Fewer fish means less ammonia.
- Stop overfeeding your fish. Less uneaten food and less fish poop means less ammonia.
-
- Remove dying plant leaves. Fewer rotten leaves in your aquarium means less ammonia.
How to check ammonia levels in a fish tank without a kit? Answer:
Test kits are really cheap but if you really don’t want to spend the $5 or $10 to buy a kit, you can have your water tested where you buy your fish. Call the store first to make sure they provide this service.
I had an ammonia spike in my cycled aquarium. What happened? Answer:
Probably one of two things happened.
1. Someone (not you) fed your fish. Overfed actually. (Not very likely)
2. You had a fish die and you didn’t notice until you had an ammonia spike (likely). See my story about not finding a dead snail in this article.
Best Fish For Beginners


Categories: small fish, beginner fish, easy fish
Ammonia in an aquarium:
The Invisible Threat: Keeping Your Aquarium Safe from Ammonia and Nitrite
I’ve always loved aquariums. The calming presence of fish gliding through a miniature underwater world is truly mesmerizing. But setting up my first tank was a rollercoaster ride. Sure, the décor was perfect, the fish looked vibrant, but something lurked beneath the surface – ammonia and nitrite. Nasty stuff, turns out, deadly to fish even in small amounts.
The Silent Danger: Ammonia and Fish Waste
Imagine my horror when I learned this invisible threat came from my fish themselves! Their waste breaks down into ammonia, which can burn their gills and cause organ damage. Yikes! Thankfully, there are these superhero bacteria that take up residence in the aquarium filter. They’re the nitrifying bacteria, and they gobble up the ammonia, converting it into something a little less scary – nitrite.
Building an Army: The Importance of Tank Cycling
But here’s the catch: these superhero bacteria need time to establish their colony. You can’t just dump a bunch of fish in and expect them to thrive. That’s where the magic of tank cycling comes in. It’s basically giving the good bacteria a head start by letting them grow in the tank without any fish. There are different ways to cycle a tank – you can add a sprinkle of ammonia yourself, or even borrow some filter media from a friend’s established tank to jumpstart the process.
Testing the Waters: Monitoring Ammonia and Nitrite Levels
The key during this cycling period is patience and vigilance. You become a water detective, constantly testing the levels of ammonia and nitrite. It’s like watching a race – the ammonia levels rise first, then the nitrite, as the different bacteria take over. But after a few weeks, things settle down. The good bacteria become a strong army, keeping ammonia and nitrite in check, making the tank a safe haven for your future finned friends.
Welcome Home, Fish! Maintaining a Healthy Aquarium
Finally, the moment arrives – adding fish! But remember, it’s not a free-for-all. Overcrowding or overfeeding can overwhelm the superhero bacteria, sending those ammonia levels spiking again. So regular water changes become your new routine, kind of like cleaning your house to keep it healthy.
Keeping an Eye Out: Monitoring Water Quality
Testing the water becomes second nature. Any traces of ammonia or nitrite are a red flag, prompting an investigation into my aquarium maintenance. Maybe I need a bigger filter, a water change, or to lay off the fish food for a bit. But the good news is, with a little care and attention, I can create a sparkling clean underwater world where my fish can thrive.
The Final Piece: Understanding Nitrate
There’s also this third player in the nitrogen cycle – nitrate. It’s the leftover product after the bacteria finish with the nitrite. Not as harmful, but still something to keep an eye on with water changes or plants that absorb it.
A Well-Kept Secret: The Danger of Well Water
And one last thing – well water can be a sneaky culprit. It might have too much nitrogen gas, which can be dangerous for fish. So, if I’m using well water, I have to take an extra step to de-gas it first.
The Reward: A Thriving Underwater World
All in all, keeping an aquarium is a balancing act. But by understanding the nitrogen cycle and becoming a water detective, I can create a thriving underwater world for my finned friends. It’s a constant learning process, but the reward – a healthy, vibrant aquarium – is absolutely worth it.
Copyright information for the following important article:
The documents contained on this website are copyrighted by the University of Florida, Institute of Food and Agricultural Sciences (UF/IFAS) for the people of the State of Florida. UF/IFAS retains all rights under all conventions, but permits free reproduction by all agents and offices of the Cooperative Extension Service and the people of the State of Florida. Permission is granted to others to use these materials in part or in full for educational purposes, provided that full credit is given to the UF/IFAS, citing the publication, its source, and date of publication.
Some images, publication thumbnails in particular, may have been generate using AI (DALL-E 2)
The original article location can be found here: https://edis.ifas.ufl.edu/publication/FA031
Ammonia in an aquarium: Part 3
From IFAS Extension – University of Florida
AMMONIA IN AQUATIC SYSTEMS
By: Ruth Francis-Floyd, Craig Watson, Denise Petty, and Deborah B. Pouder
INTRODUCTION
All animals excrete waste in the process of metabolizing food into the energy, nutrients, and proteins they use for survival and growth. In fish, this primary metabolic waste product is ammonia.
In fish, the majority of ammonia is eliminated from the body primarily by diffusion through the fish’s gills into the water. Smaller amounts are excreted in the urine or across other tissues.
Fertilizers and the decay of uneaten feed and organic matter contribute to ammonia, but in most aquaculture or hobbyist systems, the digestion of the feed eaten by the fish is the primary source of the compound. The more feed a fish is fed, the more ammonia the fish will produce. However, even a starved fish will produce some ammonia.
Of all the water quality parameters that affect fish, ammonia is the most important after oxygen, especially in intensive systems. At low concentrations, ammonia causes stress and damages gills and other tissues. Fish exposed to low levels of ammonia over time are more susceptible to bacterial infections, have poor growth, and will not tolerate routine handling as well as they otherwise would. At higher concentrations, ammonia will kill fish, and many unexplained production losses have likely been caused by ammonia.
Ammonia accumulates easily in aquatic systems because it is a natural byproduct of fish metabolism. Because it is continuously excreted, producers and other fish owners must regularly measure ammonia and eliminate it from systems before it can accumulate and damage or kill fish. Additionally, ammonia may be present in source waters such as some municipal (city) or well waters.
Ammonia is colorless and, even at levels toxic to fish, odorless. Therefore, the only way for a producer or aquarist to know if ammonia is present is to test the water.
In water, ammonia exists in two chemical forms: un-ionized ammonia (NH3) and ionized ammonium (NH4+). The combined concentration of these two forms is called total ammonia or total ammonia nitrogen (TAN). The portion of the total ammonia in the un-ionized or ionized forms is affected primarily by pH and temperature and, to a lesser extent, salinity. The portion of un-ionized ammonia increases with increasing pH and/or temperature but decreases slightly with increasing salinity. It is important to know the portion of ammonia in the un-ionized form (NH3) because it is approximately 100 times more toxic to fish than the ionized (NH4+) form.
THE NITROGEN CYCLE
A biological process called the nitrogen cycle (or nitrification) eliminates ammonia from water by converting it to a less toxic compound through a series of reactions. The ammonia excreted by fish is converted to a compound called nitrite (NO2-) by several genera of bacteria, including Nitrosospira and Nitrosomonas. As a group, these bacteria are called ammonia-oxidizing bacteria (AOB). Other groups of bacteria, including Nitrospira and Nitrobacter (and collectively called nitrite-oxidizing bacteria [NOB]) convert nitrite to the much less toxic nitrate (NO3-). Nitrification is an aerobic (oxygen required) process that results in the production of carbon dioxide (CO2) and free hydrogen ions (H+), reducing the pH of the water unless sufficient buffers (alkalinity) are present.
In ponds, the nitrification process takes place on the surface layers of the mud/substrate and on plants or other structures where the nitrifying bacteria colonize. In tanks or aquaria, a biological filter (“biofilter”), must be provided as a place where the bacteria can colonize and flourish. A new biofilter requires six to eight weeks to build up sufficient bacteria to effectively reduce ammonia and nitrite levels.
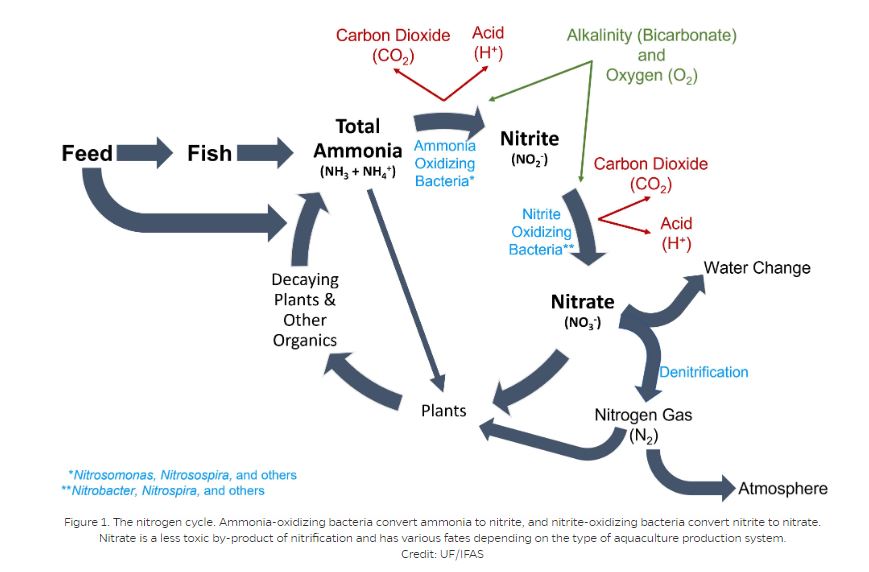
The nitrogen cycle. Ammonia-oxidizing bacteria convert ammonia to nitrite, and nitrite-oxidizing bacteria convert nitrite to nitrate. Nitrate is a less toxic by-product of nitrification and has various fates depending on the type of aquaculture production system.
Figure 1. The nitrogen cycle. Ammonia-oxidizing bacteria convert ammonia to nitrite, and nitrite-oxidizing bacteria convert nitrite to nitrate. Nitrate is a less toxic by-product of nitrification and has various fates depending on the type of aquaculture production system.
Credit: UF/IFAS
Nitrite is toxic to fish at levels as low as 0.10 mg/L. Traditionally, nitrate has been considered non-toxic to fish even at levels up to 200 mg/L. However, more recent research is showing nitrate may be more detrimental than previously believed. In natural systems and ponds, nitrate is taken up as a fertilizer by plants, including phytoplankton, so levels typically remain low. In closed systems with little or no water exchange, however, nitrate can accumulate to higher than 250 mg/L. Water changes or separate denitrification systems are needed to remove nitrate from recirculating system water.
AMMONIA TESTING
All aquaculturists and hobbyists should invest in a dependable water quality test kit. A good water quality management program will reduce fish disease problems, promote growth, and lessen the need for chemical treatments. A water quality test kit will pay for itself many times over, both in numbers of fish saved and increased production.
Most commercial ammonia test kits measure the total ammonia nitrogen (TAN) in milligrams per liter (mg/L), which is the same as parts per million (ppm). Again, it is the un-ionized ammonia (or UIA) portion of the TAN that is more toxic. To determine the UIA fraction from the TAN measurement, the temperature and pH of the water must also be known. At high temperatures and high pH, there is more UIA. Therefore, a good ammonia test kit will include a TAN test, a pH test, and a thermometer.
There are two common types of ammonia test kits, and each uses a different testing method to determine TAN. One is the Nessler’s method and the other is the ammonia salicylate method. If formalin or formalin-containing products have been used in the water within 24–72 hours, the Nessler’s method will result in a falsely elevated ammonia reading. Use of ammonia binding products and many “water conditioners,” such as those used in pre-treating municipal water sources, will also cause false high ammonia readings with the Nessler’s method. This method also produces false reactions when used to test seawater. The reagent used in the Nessler’s method contains a small amount of mercury that in many states must be disposed of as hazardous waste.
The other testing method is the ammonia salicylate method. This method is not affected by ammonia binding products or formalin treatments. The ammonia salicylate method is also more accurate than the Nessler’s method when testing ammonia in seawater.
WHEN SHOULD AMMONIA BE TESTED?
In general, ammonia should be tested once a week in any production system. Ammonia (and other water quality parameters) should also be tested immediately any time fish appearance or behavior changes (including reduced feeding).
If multiple tanks depend upon a common biofilter (i.e., a recirculating system), there is no need to check every tank individually. A good practice is to consistently test the ammonia level in the tank with the highest feeding rate and spot test other tanks from time to time. Although a system shares a common filtration system, there can be some differences in water quality between tanks. If fish show any signs of abnormality, water quality should be tested immediately in that individual tank.
Keep records for all tests and monitor trends. Whenever ammonia is found, increase the frequency of testing until the problem is corrected. As mentioned previously, whenever fish are sick, test the water quality immediately.
Ammonia is likely responsible for more unexplained losses in aquaculture than any other water quality parameter. Because it is colorless and odorless at levels toxic to fish, the only way to know if it is present is to test for it. Fish submitted to a diagnostic laboratory are often tested for infectious diseases (bacteria, parasites, fungi or viruses) only. Even if a water sample is submitted to the laboratory for testing, it provides only a one-time evaluation that provides no insight into past trends. It is the responsibility of producers and aquarists to regularly test the water quality, which is very often the underlying problem. Fish may become sick even weeks after a water quality problem has been corrected, so a single, lab-tested sample cannot be used to fully evaluate the water quality effect on a fish disease problem.
INTERPRETING THE AMMONIA TEST
In healthy ponds and tanks, ammonia levels should always be zero. Presence of ammonia is an indication that the system is out of balance. Therefore, any ammonia in a pond or tank should alert the producer or aquarist to examine system management and to start corrective measures.
To determine UIA, three tests must be performed: TAN, pH, and temperature. Once these three parameters are measured, the fraction of UIA can be calculated using a multiplication factor found in Table 1. Find the measured temperature on the top row of the table and the measured pH in the left column. The number at which the appropriate column and row intersect in the table is multiplied by the measured TAN to give the UIA in mg/L. If temperature or pH falls between values on the table, round up to the next highest value to determine the “worst case scenario” because higher pH and temperature result in more UIA.
Anytime the UIA is higher than 0.05 mg/L, damage to fish tissues can occur. As the concentration rises above 0.05 mg/L, it causes more and more damage. At 2.0 mg/L, sensitive fish will typically die. Even if fish do not die directly from high ammonia levels, they become more susceptible to infectious disease. Again, any ammonia indicates a problem in your system. If you find it, take corrective measures immediately.
MANAGEMENT OF AN AMMONIA PROBLEM
The first thing to do when ammonia is present in a pond or tank is to reduce or stop feeding. Fish may not eat during periods of ammonia stress, and the uneaten feed will only make the situation worse. Reducing or stopping feed for a short period of time (e.g., 1–3 days) will not typically have a negative effect on fish (except fry). Overfeeding is a major cause of high ammonia concentrations, and stopping the feeding will allow the nitrogen cycle to “catch up” with the nutrient load. A 25% to 50% water change will help remove some ammonia, assuming the incoming water source does not contain ammonia. This process is only feasible in tanks or very small ponds, so do not try to solve an ammonia problem in a large pond using this method.
Low levels of dissolved oxygen limit the ability of nitrifying bacteria to convert ammonia and nitrite, so it is important to monitor dissolved oxygen and take steps to increase oxygen if it falls below 5 mg/L
In ponds, the addition of a phosphate fertilizer may help to relieve high TAN levels over a period of days by stimulating phytoplankton growth, which helps remove ammonia from the system; however, it may not help quickly enough in an acute ammonia crisis. Use a 0–20–0 fertilizer at a rate of 40 pounds per acre. It is important not to use a fertilizer that contains nitrogen because nitrogen will add to the problem. If phosphorus is not a limiting factor for algal growth in the pond, the phosphate fertilizer method will not work at all. Similarly, if the pond already has a heavy phytoplankton bloom, fertilizer should not be added.
In tank systems, water changes and the use of ammonia-binding products can alleviate ammonia toxicity in the short term. However, for long-term management, it is best to establish a properly designed and sized biofilter.
In tanks without a biofilter, the producer or aquarist should strongly consider incorporating one. Given the six to eight weeks necessary to establish a biofilter, this will not help in a crisis, but it is a long-term solution to the problem.Biofilters should be sized appropriately according to feed input.
With the exception of high-flow raceways, such as those used in the salmonid aquaculture industry, many flow-through tank systems do not have sufficient flow to eliminate ammonia adequately, so testing and management of ammonia in these systems is still critical.
Some chemicals used to treat diseases in fish, especially antibiotics, can be detrimental to the nitrifying bacteria in the biofilter. Both ammonia and nitrite levels should be tested more frequently after applying a disease treatment to ensure that the biofilter is still functioning appropriately.
SUMMARY
Ammonia is a major waste product of fish and is also produced as a breakdown of feed and other organics. It can accumulate in aquaculture or aquarium systems, where it will, at the very least, decrease production. It is frequently a stressor that leads to disease, and in other cases it kills fish directly. The only way to detect its presence is to test for it. A fish farmer or aquarist should invest in a dependable water quality test kit, learn how it works, and use it regularly.
Ammonia test kits only measure the total ammonia nitrogen (TAN). When this test indicates a reading above zero, producers or aquarists can determine the amount of the more toxic un-ionized ammonia (UIA) after measuring pH and water temperature. The multiplication factors are found in Table 1, and an example calculation is found in Figure 3. When ammonia is present, the fish in the system should not be fed (or feed should be reduced) until the problem is corrected. In small systems, a water change may help, and in large ponds, a 0–20–0 fertilizer may help.
Test for ammonia regularly and take corrective measures as soon as you detect it. Severe problems may occur when tests are not performed frequently enough. Even after an ammonia problem has been corrected, fish may become sick weeks later. Once fish begin to die, it is difficult to correct an ammonia problem without losing more fish.
RECOMMENDED
Fish Health Management Considerations in Recirculating Aquaculture Systems—Part 1: Introduction and General Principles (UF/IFAS Circular 120) https://edis.ifas.ufl.edu/FA099
Southern Regional Aquaculture Center publications, available at https://srac.tamu.edu/
Ammonia in Fish Ponds (SRAC Publication No. 463)
Nitrite in Fish Ponds (SRAC Publication No. 462)
Managing Ammonia in Fish Ponds (SRAC Publication No. 4603)
How to Start a Biofilter (SRAC Publication No. 4502)
Recirculating Aquaculture Tank Production Systems: An Overview of Critical Considerations (SRAC Publication No. 451)
Recirculating Aquaculture Tank Production Systems: Management of Recirculating Systems (SRAC Publication No. 452)
Recirculating Aquaculture Tank Production Systems: A Review of Current Design Practice (SRAC Publication No. 453)
REFERENCE
Emerson, K., R. C. Russo, R. E. Lund, and R. V. Thurston. 1975. “Aqueous Ammonia Equilibrium Calculations: Effects of pH and Temperature.” Journal of the Fisheries Research Board of Canada 32:2379–2383.
Table 1. Fraction of un-ionized ammonia in aqueous solution at different pH values and temperatures. Calculated from data in Emerson et al. (1975). To calculate the amount of un-ionized ammonia present, multiply the total ammonia nitrogen (TAN) by the appropriate factor selected from this table using the pH and temperature from your water sample


